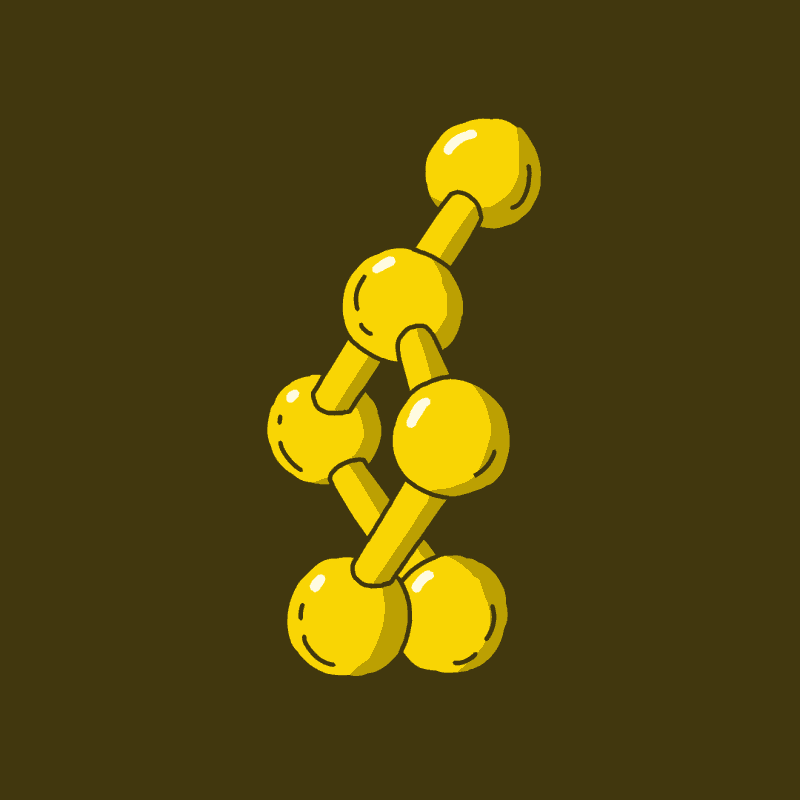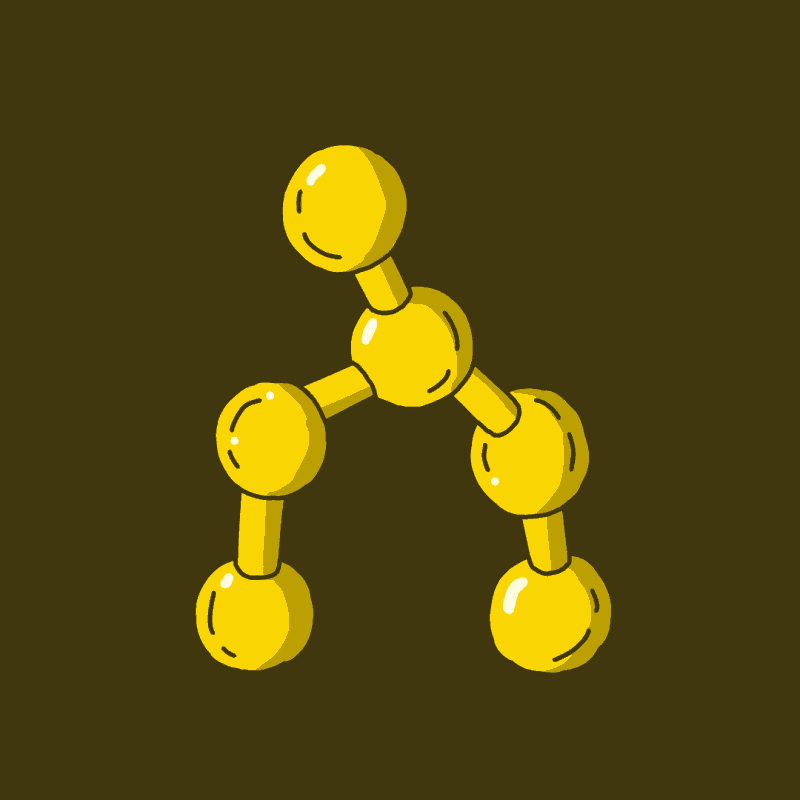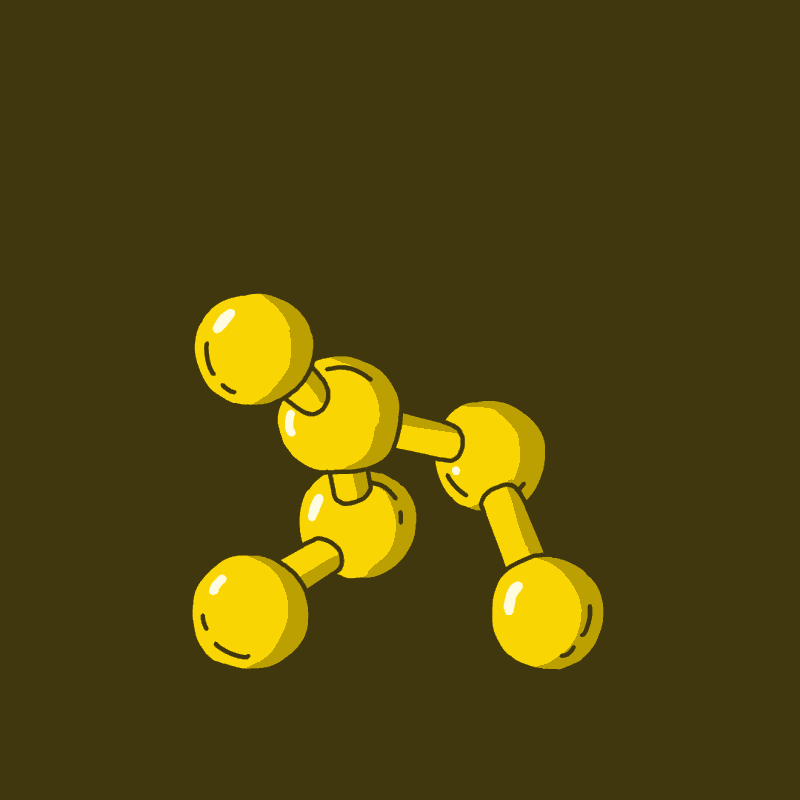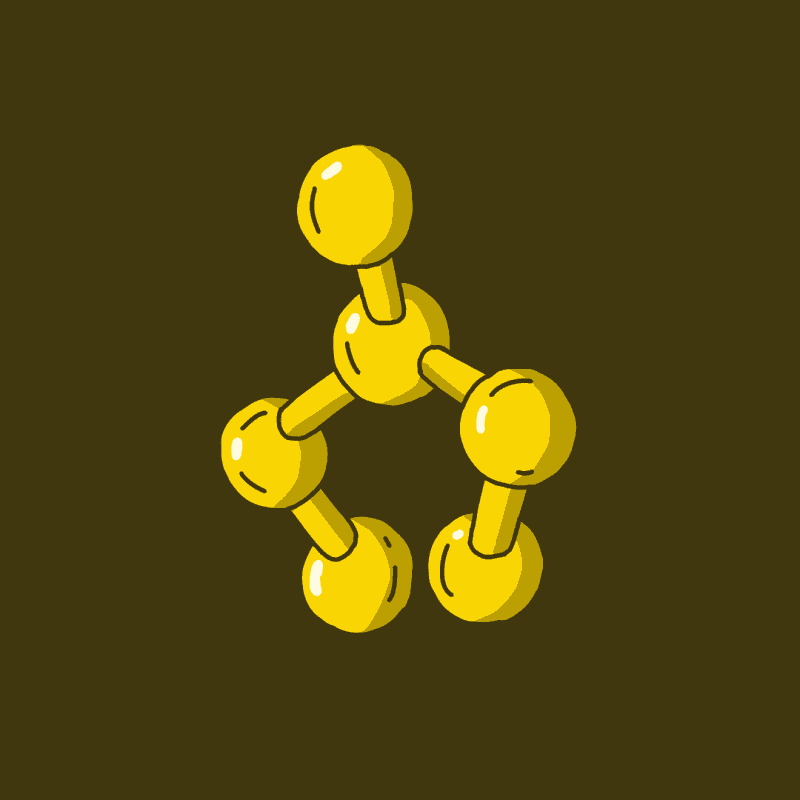The Chemistry of Psychedelics
In today’s newsletter you will learn:
Why different molecules have different properties
How the structure of psychedelic molecules contribute to the psychedelic experience
How to predict potency and hallucinogenic activity of a tryptamine based on its structure
Once upon a time…
You find yourself in the humid rainforests of the Pacific Northwest during spring, bearing witness to a dazzling array of fungal diversity littered among the paths, brush, rocks, and trees. At first you notice the bright orange chanterelles reaching out of the moist soil, subtle lavender Wood Blewits peaking out behind a mossy boulder, and waves of yellow-tipped Chicken-of-the-Woods bursting forth from the trees. If you’re lucky you might find a fly agaric mushroom. Scientifically known as Amanita muscaria, this mushroom contains a dissociative compound called muscimol, and is thought to have played a large role in the myths of early Siberian and European cultures.
The psychedelically minded will be on the lookout for Psilocybe cyanescens. Tis’ but a small mushroom with a wavy brown cap and white stem which bruises blue to the touch. Many mushrooms look similar but are poisonous, such as certain Galerina species. Plucking a candidate toadstool, you monitor the blue reaction, and carefully inspect it for the other features that will differentiate it from its deadly look-alikes. Once identified, you chomp into its earthly flesh. About thirty minutes later, the warm magic mushroom lens nestles in upon your consciousness. The trip has begun.

The Chemistry of Psychedelic Molecules
The unassuming mushroom you’ve joyfully consumed contains a compound called psilocybin. When psilocybin reaches your stomach and bloodstream, it is converted into psilocin, which is the molecule that enters the brain. Psilocin is one of many psychedelic molecules that has just the right shape to tweak the circuits in your head jelly, thereby producing a non-ordinary state of consciousness. Much of the mystique surrounding the psychedelic experience lies in the realm of biology and psychology. Before we get to that, we need to know how and why psychedelic molecules tweak those circuits, and so today we journey to the realm of chemistry.
First stop
What is a molecule? (I told you we’re starting from the ground up) A molecule is made up of atoms. Atoms are the smallest unit of matter that form an element. Oxygen, gold, and ytterbium are examples of elements. Elements have certain physical properties based on how many protons, neutrons, and electrons they have. For example, alkali metals such as sodium, lithium, and caesium have an odd number of electrons, which contributes to their high reactivity. When you arrange multiple atoms into a molecule, you get a larger structure with properties determined by the types, amounts, and arrangements of the atoms that compose it.
All living things are made up of molecules. If you wanted to get real weird with it, you could say we are just really big molecules walking around and shitting and stuff. Actually you can’t say that, I just didn’t want to delete that sentence. Anyway, a lot of the molecules in our bodies share similar arrangements of atoms, and therefore have similar, distinct properties. When we talk about psychedelic molecules, we often classify them according to the atomic arrangements they share. And so now we can talk about them!
Classifying Classical Psychedelics
Note: There are a LOT of psychedelic molecules, because there are a LOT of potential variations to the basic structure of psychedelic molecules. I can’t wait to talk about all of them. However, for the sake of teaching fundamental chemistry, I am going to stick to the most popular classical psychedelics here.
There are two main classes of psychedelics you will most often hear about in the context of scientific research, for the treatment of mental illness, and from your friend who has totally watched every episode of Hamilton’s Pharmacopeia. Those two classes are the tryptamines and phenethylamines. Tryptamines are so called because they share the ring system in Fig 1a, which is derived from the amino acid tryptophan. Phenethylamines are so called because they share the ring system in Fig 1b. Phenethylamine is named for its chemical structure, namely, a phenyl group (red) attached to an ethylamine group (blue).

The psilocin seeping through your brain after a magic fungal ingestion is a tryptamine. DMT, a psychedelic found in many plants and animals including mammals, and one of the primary active compounds in ayahuasca, is also a tryptamine. LSD is technically not a tryptamine, but a synthetic lysergamide, which is closely related. We can group them together for our purposes.
Structure, function, and alteration of consciousness
Our bodies also make different types of tryptamines, referred to as endogenous tryptamines. One of the most important tryptamines in our brains, and a crucial detail to this story, is a neurotransmitter called 5-hydroxytryptamine, i.e. *drumroll* serotonin. Serotonin is the primary neurotransmitter used by neurons in regions of the brain regulating mood, cognition, reward, learning, and memory. These neurons are referred to as serotonergic.

When a serotonergic neuron receives a signal, it releases serotonin, which then binds to serotonin receptors on neighboring neurons, effectively spreading the signal (more on this in next week’s Biology of Psychedelics newsletter). Receptors are large molecules that are built with specifically-shaped regions for molecules to bind to. Only molecules with a specific shape can activate the receptor, sort of like a key to a lock. Usually serotonin is the only molecule that can bind to these receptors. However, the spirited Psilocybe swallower will be introducing psilocin to this system, which looks very similar to serotonin (Fig 2). Psilocin can thus activate serotonergic neurons in patterns which coalesce into a psychedelic extravaganza.
Naturally, the story gets a bit more complex, but you’ve got this.
There are countless different serotonin receptors attached to a wide range of neuron types spread across many unique regions of the brain. Tryptamines primarily activate a receptor called serotonin HT2A receptor. However, tryptamine molecules are promiscuous and tend to activate a bunch of other receptor types around the brain to a lesser degree. Now, as I said before, subtle differences in molecular structure cause differences in a molecule’s chemical properties. So, the different structures of tryptamine molecules have different tendencies to activate these other receptors in the brain. So while psilocin, DMT, and LSD all activate serotonin HT2A receptors, they each also activate their own unique subset of other receptors around the brain as well.
The diversity of receptors, neurons, circuits, and brain regions that can influenced in different ways by different tryptamines are all considered to contribute to the diversity of psychedelics experiences possible.
Real chemistry for real psychonauts
Before we finish, I want to share how a few specific modifications to the basic tryptamine molecule impacts their effects. The most important tryptamine structure for trippin’ is the indole (Fig. 3a, red). Note the numbers and greek letters around the molecules in Fig. 3. These are all positions where the molecule can be modified. Both publicly-funded and clandestine drug design chemists have spent many hours testing tryptamine derivatives in animal models, muscle tissue, and on themselves. By modifying the indole at the 6 & 7 position, the hallucinogenic activity is decreased, and so molecules with these modifications are rarely produced for recreational purposes. Adding a hydroxyl group at the 4 position, or a methoxy at the 5 position increase potency above other substitutions at these positions (Fig 3b & 3c). Potency refers to the amount of a substance needed to induce the desired affect.

I haven’t talked much about phenethylamines, as I am going to focus on them more closely in next week’s Biology of Psychedelics newsletter. However, I want to mention them here to demonstrate that it is not just the receptor-binding affinity that contributes to the effect of the drug. Adding a methyl group to the amine group and the alpha-carbon on the phenethylamine group does two things; 1) it increases the ability for the molecule to cross the blood-brain barrier and 2) it prevents the molecules from being degraded by enzymes in our stomach. Together, these features increase the effect of the drug by increasing the amount that can reach receptors in the brain.
These few examples are a drop in the pond of potential structure-function relationships in psychedelic molecules. Just like a modification can increase the psychedelic activity of a molecule, it can also increase the toxicity. Always do your due diligence when researching the main effects and side effects of drugs, and if someone hands you an obscure tryptamine derivative at a party or festival, test yo’ shit.
Further Reading
📚 PiKHAL: A Chemical Love Story and it’s sequel, TiKHAL: The Continuation by Alexander and Ann Shulgin are two of the most well-known books among psychonauts. PiKHAL stands for Phenethylamines I Have Known And Loved, and TiKHAL stands for the same, subbing in Tryptamines for the T.
The books contained detailed chemical synthesis techniques for hundreds of phenethylamine and tryptamine derivatives. It also details the clandestine chemist couple’s love life, enhanced by their concoctions. It is some quality interdisciplinary research.
From around the psychosphere
🧪 Science & Culture:
30 minute podcast episode with insight into participating in a psychedelic study: How Psychedelic Drugs Are Making a Comeback to Treat Depression
⚖️ Ethics:
In-depth look at the intersection of psychedelics and far-right conspiracy politics, i.e. “conspirituality”: Turn Off Your Mind, Relax—and Float Right-Wing?
💸 Finance:
Debate is raging about the current psychedelic patent landgrab. Get the lowdown here: Investors are Debating Who Should Own the Future of Psychedelics
Stay up on psychedelic finance here: PsilocybinAlpha
Follow:


Tunes for your next flow state:
🔥 Greta Van Fleet - Live at Red Rocks
🍯 Jhené Aiko — Tiny Desk (Home) Concert
⚗️ Stay tuned Friday for a breakdown of this weeks psychedelic research article
🌵 Dropping next Monday: The Biology of Psychedelics