A non-hallucinogenic psychedelic analogue with therapeutic potential; Cameron et al. 2021
Friday Journal Club #5
Few things before we begin:
Spring is in the air, go take a walk and breathe it in!
In the words of myself, “your follows, shares, comments, and likes will help this nubile publication transcend the dregs of internet algorithmic wastelands to ultimate interweb glory. If you like my stuff, smash that like button, comment your thoughts and opinions, and follow me on Twitter @TyQuig and Instagram @thetab_psychedelicscience”
Part of this newsletter is 24/7 office hours. If you have a question about psychedelic science, send it my way.
Now on to…
A non-hallucinogenic psychedelic analogue with therapeutic potential
Why they did it:
There is some evidence (anecdotal and open-label studies) that ibogaine may be a potent addiction treatment, though it’s exact mechanism is unknown.
Evidence from rodent models suggest ibogaine promotes neuroplasticity and signaling pathways in regions of the brain related to addiction behaviors. 🧠
Ibogaine is converted to noribogaine in the body. Noribogaine is a strong psychoplastogen, a class of molecule that promotes neuroplasticity. Noribogaine increases the complexity of the neuronal dendrite patterns.
Since psychoplastogens alter brain connectivity rather than just blocking the targets of addictive drugs, they could reduce proclivity for addiction itself, rather than addictions to specific substances.
Despite it’s promise, ibogaine has not been tested in double-blind, placebo controlled studies necessary for FDA approval. 🙅🏽♂️
Supply of ibogaine is limited by overexploitation of the plant it is found (Tabernanthe iboga), and by lack of a synthesis technique for consistent, large-scale production. 🌳
Ibogaine has a poor safety profile, largely due to its non-polar nature. Its non-polarity causes it to accumulate in fat tissue and have toxicity to the heart. ⛔️🫀
A molecule with the same (potential) anti-addictive properties of ibogaine, that is also safe and can be produced at scale, could be an extremely useful molecule for treating addiction. Also, this would limit the conservation threat to Tabernanthe iboga.

What they did:
Identified the key structural features of ibogaine, synthesized a variety of ibogaine-like molecules by removing structural features in different combinations, and then tested their psychoplastogenic properties. ⚛
After identifying the molecule with the highest psychoplastogenic properties and likelihood for an improved safety profile, they noticed that it resembled 5-MeO-DMT. Since 5-MeO-DMT is hallucinogenic, but 6-MeO-DMT is not, they altered their molecule to resemble 6-MeO-DMT.
They named this molecule tabernanthalog (TBG), tested its hallucination-producing potential, and examined its safety profile through a variety of tests on zebrafish (detailed below). Zebrafish are a common model for collecting pre-clinical data on drug safety. 🦓🐟
They then tested TBG for it’s binding properties in the brain, as well as its effect on neural plasticity, anti-depressant behavior, and alcohol- and heroin-seeking behavior.

What they found:
TBG is non-hallucinogenic 🤗
Researchers gave mice TBG and quantified their head-twitch behavior, a behavior associated with hallucinations. More head-twitch = more hallucinogenic potential.
Per this test,TBG is significantly less hallucinogenic than positive control 5-MeO-DMT, a highly hallucinogenic psychedelic.
TBG is safer than ibogaine 🦺
Ibogaine inhibits an important channel in the heart, which can cause cardiac arrest. The authors found that over 100x more TBG is needed to inhibit this same channel.
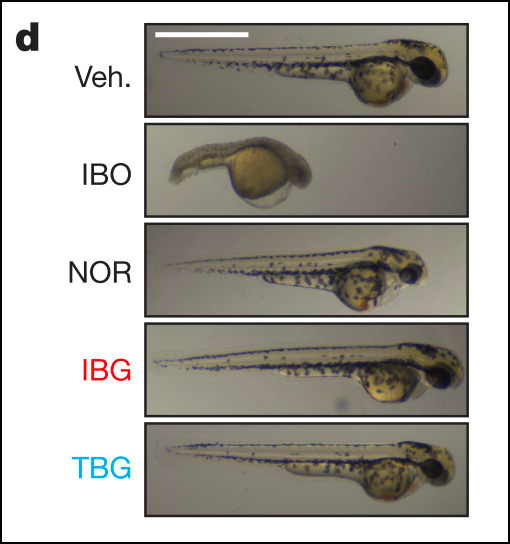
Larval zebrafish dosed with ibogaine had a slowed heart rate and increased chances of cardiac arrhythmia, whereas TBG-dosed zebrafish had neither.
Neither ibogaine nor TBG induced seizures in larval zebrafish.
Ibogaine treatment significantly increased malformations and death in larval zebrafish over control, while TBG treatment caused significantly less malformation and death than ibogaine. When the concentration of TBG administered was reduced from 100 𝜇M to 66 𝜇M, there was no difference between TBG treatment and control.
TBG targets serotonin 5HT2a receptors 🎯
TBG shows little to no activity at opioid receptors
TBG activates 5HT2a receptors, and blocks serotonin 5HT2b receptors
TBG promotes neural plasticity in rat embryonic cortical neurons 🐁
TBG increased dendritic complexity
Dendrites are the part of the neuron that receives signals from other neurons
Complexity is measured by placing a series of concentric rings around an image of a neuron and counting the number of times dendrites cross the rings. See more here.
TBG increases dendritic spine formation and density
Spines are small protrusions where dendrites receive signals

TBG has anti-depressant effects 😊✅
Researchers put mice through a forced swim test, in which a mouse is placed into an inescapable tank of water for a period of time, and the amount of time the mouse remains immobile is assessed.
Immobility in the water is viewed as a measure of ‘despair’ or ‘negative mood’. This test is commonly used to test anti-depressants, which usually reduce immobility time.
The researchers mildly stressed out mice for 7 days, which increased their immobility time in the forced swim test. Some of these mice subsequently received a dose of TBG, which reduced their immobility time.
Treatment with ketanserin, a molecule that blocks serotonin 5HT2a receptors, eliminated the effect of TBG in the forced swim test.
TBG reduces alcohol dependence ❌🥃
Researchers put mice through a protocol that hooks the mice on booze. When they injected TBG into some of the mice, those mice drank less alcohol (but importantly, not less water). For the next two days, these mice drank less alcohol than control.
They repeated this experiment using sugar water, to no effect. This shows that TBG is selective for reducing alcohol consumption.
TBG reduces heroin dependence ❌💉
Researchers let mice have free access to heroin, then removed heroin access, then reinstated it. They administered TBG to mice at each of the stages, and found that, at each stage, these mice sought out the heroin less than control.
The mice that received TBG during the administration phase also had reduced heroin-seeking behavior during the final reinstatement phase, suggesting the anti-addictive effects of TBG may last up to 14 days.
They repeated this exact test with sugar, and found that TBG induced the same effects. This might suggest that TBG is not selective for heroin, but disrupts reward learning in general.
My Take:
For some reason, academics and industry folk are really obsessed with finding ways to take the fun out of psychedelic molecules. Nah I am being facetious. Removing the hallucinogenic aspect of these compounds can help alleviate serious ailments without the commitment of a 12-24 hour inner journey. The authors of this paper have done their due diligence in testing TBG’s potential to be a safe alternative to the ibogaine. Further work in humans is certainly needed, and is likely forthcoming, as the senior author of this paper, David Olson, cofounded a company to take TBG and other psychoplastogens through FDA clinical trials.
That being said, there is debate on the importance of the subjective experience of the trip for therapeutic benefits. See this paper and this debate between David Olson and one of its authors.
I appreciate your feedback on how I did breaking down this science. Let me know in the comments:
Think more people should know about psychedelic science? Share my newsletter with your people, because your people are my people ✌🏽.
📃 Here’s the paper:
Cameron, L. P. et al. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature. 589, 474–479 (2021).
From around the psychosphere:
🔊 Here is a great episode from the Bio Eats World podcast with senior author Dr. David Olson about this paper and the challenges in the psychedelic medical field
📰 TIME article: Inside Ibogaine, One of the Most Promising and Perilous Psychedelics for Addiction
🎥 Learn more about ibogaine and opioid addiction in this TED Talk by ibogaine researcher Thomas Kingsley Brown:
👩🏾💻 Follow the Olson Lab on Twitter:


🧠 Happy Friday, see you Monday for The Psychology of Psychedelics 🔬







Well written, Tyler. Keep up the good work! I look forward to your next release.